Adapted from: Peter M. Bierman and Carl J. Rosen. Nutrient Cycling and Maintain Soil Fertility in Fruit and Vegetable Crop Systems. Department of Soil, Water and Climate, University of Minnesota. Available at: http://www.extension.umn.edu/distribution/horticulture/m1193.html#nutcyc
Introduction
Plants require four factors for growth and reproduction: light, water, the right temperature, and nutrients. Plant nutrients are chemical elements that are mostly absorbed by plant roots as inorganic chemicals dissolved in water, and it is important to understand both the biological and chemical processes that provide plants with nutrients. Nutrients needed by plants are used by other forms of life and go through many biological transformations that determine when and how plants take them up. Biological materials like leaf litter or animal waste are major nutrient sources in forest ecosystems.
Plant Nutrients
Plants need at least 16 essential chemical elements for growth. Carbon, hydrogen, and oxygen, obtained in large amounts from air and water, make up the bulk of plant dry matter in the products of photosynthesis but usually are not included as “nutrient” elements. Nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), manganese (Mn), zinc (Zn), copper (Cu), boron (B), molybdenum (Mo), and chlorine (Cl) are obtained from the soil and required by all plants. Sodium, silicon, and nickel are essential elements for some plant species and, although not required, have positive or beneficial effects on the growth of other species. Cobalt is essential for nitrogen fixation by legumes.
Sources and Losses of Plant Nutrients
Plants obtain mineral nutrients through root uptake from the soil solution. Sources of these soluble nutrients in soil include:
- Decomposition of plant residues, animal remains, and soil microorganisms
- Weathering of soil minerals
- Fertilizer applications
- N-fixation by legumes
- Atmospheric deposition, such as N and S from acid rain or N-fixation by lightning discharges
- Deposition of nutrient-rich sediment from erosion and flooding.
Mineral nutrients also can be lost from the soil system and become unavailable for plant uptake. Nutrient losses are not just costly and wasteful; they can be a source of environmental contamination when they reach lakes, rivers, and groundwater. Nutrient losses occur through:
- Runoff – loss of dissolved nutrients in water moving across the soil surface
- Erosion – loss of nutrients in or attached to soil particles that are removed from fields by wind or water movement
- Leaching – loss of dissolved nutrients in water that moves down through the soil to groundwater or out of the field through drain lines
- Gaseous losses to the atmosphere – primarily losses of different N forms through volatilization and denitrification
- Tree removal – plant uptake and removal of nutrients from the field in harvested products.
Nutrient Pools
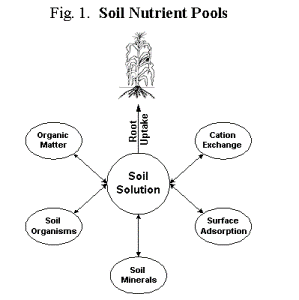
In addition to the variety of inputs and outputs, plant nutrients exist in many different forms, or nutrient pools, within the soil (Fig. 1). These pools range from soluble, readily available forms, to weakly bound forms that are in rapid equilibrium with soluble pools, to strongly bound or precipitated forms that are very insoluble and become available only over long time periods. Nutrients in solution can be taken up immediately by plant roots, but they also move with water and can easily leach below the plant root zone or be lost in runoff.
Exchangeable cations are a short-term storage pool that can rapidly replenish nutrient ions in the soil solution. Soil organic matter releases nutrients slowly as it decomposes and serves as an important supply of N, P, S, B, and trace-metal micronutrients. Soil minerals vary from relatively soluble types (chlorides and sulfates) to insoluble forms (feldspars, apatite, mica) that release nutrients through weathering reactions with chemical and biochemical agents such as organic acids. Adsorbed anions, like phosphate and iron oxides bound to clay and organic matter surfaces, are held strongly and released very slowly but can contribute to the long-term supply of plant-available nutrients.
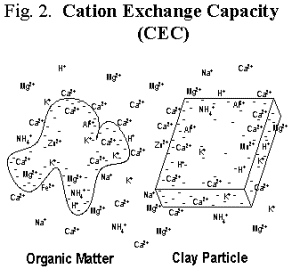
Clay particles and organic matter have negatively charged sites that hold positively charged ions (cations) on their surfaces (Fig. 2). These charged ions determine the soil’s cation exchange capacity (CEC). CEC protects soluble cations from leaching out of the plant root zone. These ions are rapidly exchangeable with other soluble ions, so when root uptake depletes the nutrient supply, they replenish plant-available cations in the soil solution. Cation exchange is the major nutrient reservoir of K+, Ca2+, and Mg2+, is important for holding onto N in the ammonium (NH4+) form, and to some extent supplies micronutrient trace metals like Zn2+ and Mn2+. Cation exchange helps soils resist changes in pH in addition to retaining plant nutrients.
Soil organic matter is a very important factor in soil fertility. It is a reservoir of plant nutrients, has a high CEC, buffers soil pH, and chelate micronutrients. Organic matter exists in different forms in soil, ranging from living soil organisms to fresh, readily decomposed plant residues, to humus that is very stable and resistant to further degradation. Living soil organisms include bacteria, fungi, actinomycetes, nematodes, earthworms, mites, and insects. They make up the soil food web, which carries out biological nutrient cycling. Plant roots are a sometimes forgotten part of the living soil biomass. Readily decomposed or active organic matter is the form of organic matter through which nutrients are actively recycled. Decomposition produces gums, polysaccharides (sugars), and other compounds that are the “glues” of water-stable soil aggregates necessary for good soil structure. Stable humus contributes to long-term nutrient supply and is the organic matter fraction with high CEC. Chelation is the ability of soluble organic compounds to form complexes with micronutrient metals that keep them in solution and available for uptake. In organic soils (peats and mucks), trace metal complexes with organic matter can reduce their availability.
The cycling of plant nutrients through soil organic matter supplies a significant portion of a growing crop’s nutrient needs. Another aspect of this cyclical process is that organic matter not only contributes to soil fertility, but fertile soils contribute to the production of organic matter.
Adapted for eXtension.org by Sabrina Kleinman, University of Arizona
For more on Dynamics of Nutrient Cycling: